
Most out of spec (OOS) deviations are not quality related
Learn more why leading pharmaceutical QC labs could split their OOS events in half!
Have you ever experienced that an OOS deviation was not due to the product quality but because of an error during the testing procedure? Well, you are not alone as the majority of OOS events are not quality related but due errors during the testing procedures. Considering the complexity of the procedures including sampling, sample preparation, analysis and documentation, the occurrence of human errors is not surprising as the steps are mostly performed manually. Sample preparation is the most error-prone process and causes around one third of the errors.
Done manually, identifying the root cause can be very time consuming. A deviation requires extensive investigations, justification, documentation, and the execution of corrective and preventive actions. During ongoing investigations und by the time the quality could be proofed to be within specifications, a batch is put into quarantine and cannot be released to the market. All this results in tremendous amount of work for the quality assurance and delays in the supply chain, which sums up to significant costs.
The quality control and -assurance groups within the pharmaceutical industry have tried to reduce OOS events applying many different approaches. Rewriting or adding details to standard operation procedures (SOPs), simplifying methods, investments in the operator’s education or reducing fluctuation did not bring the desired long-term success. Human errors frequently happen even in the simplest procedures and to the best educated lab technicians.
Regulatory compliance is the top priority in pharmaceutical manufacturing whereas deviations could lead to discarding a batch. The conduction of a quality control test procedure requires well educated professionals as well as expensive testing equipment and lab infrastructure. The high costs and the susceptibility to errors make the test procedure very cost inefficient.
The most significant reduction of human errors can be achieved by the automation of sample preparation. Automation does not only reduce human errors but also standardize sample preparation and therefore, minimize human-caused variability. Further sample preparation automation includes its documentation.
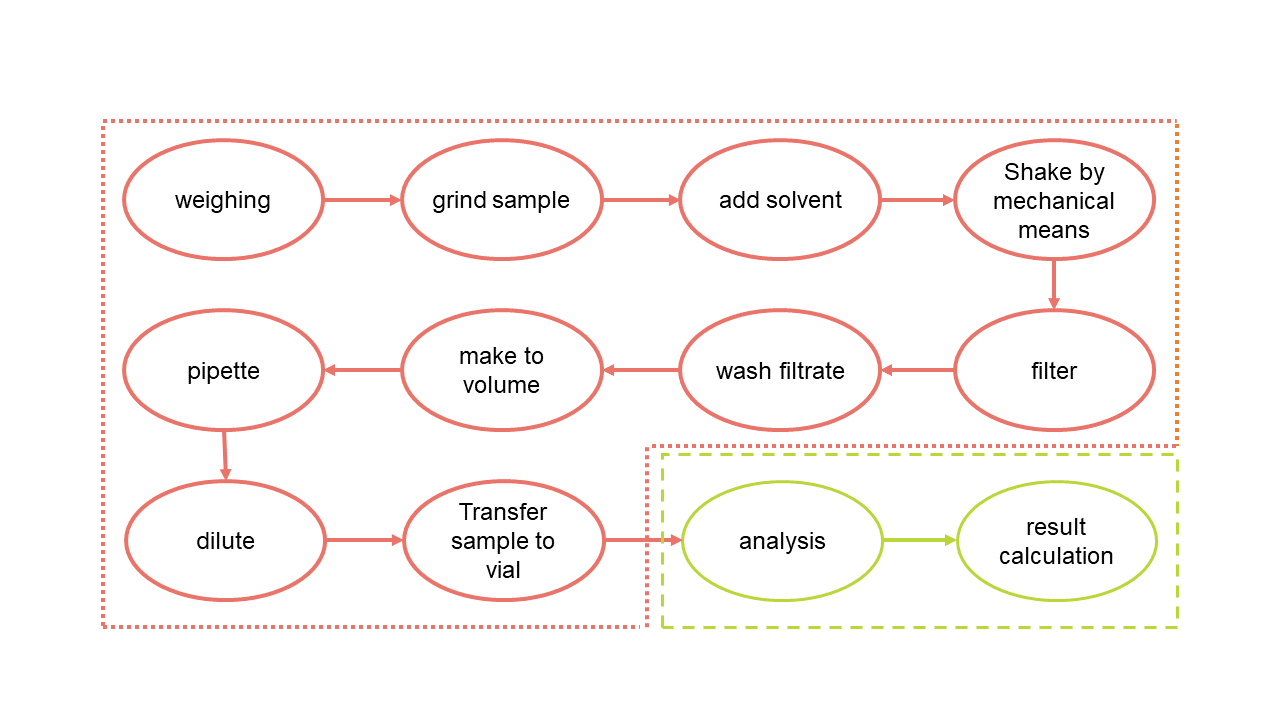
The accroma samplePrep system automates the entire sample preparation process from sample weighing until the transfer of the prepared solution into a vial or if desired, directly into a liquid chromatograph. The samples are prepared in single-use tubes and therefore, spatially separated from each other. Depending on the sample, an appropriate milling aid such as a steel ball is added. The tubes are then placed by a robot into the different modules such as the balance, shaker, liquid handler, ultrasonic bath, centrifuge, and the filtration module.
As no washing steps are necessary, the accuracy, reproducibility and flexibility of the system is outstanding. Even a single sample is worth to be prepared on the accroma. Sample preparation is made fast and standardized while the documentation is traceable and performed automatically.
The accroma samplePrep system reduce errors but also safe the lab technician’s hands-on time. The method transfer from manual sample preparation to the accroma is straight forward and may not need any adjustments. Nevertheless, methods could be optimized to reduce preparation cycle times and solvent usage. The workflow editor in the accroLab software allows simple workflow development and adjustment. The creation of workflows does neither requires any special IT skills nor is it time consuming.
The accroma system comes together with the accroLab Software, which will be installed and qualified by an accroma representative. The installation package also includes a basic training, a starter kit with consumables and 12-month warranty. Everything is included for a successful start.
Leading pharmaceutical companies could reduce hands on time by a factor of ten and significantly eliminate Out of specification events related to sample preparation. The overall reproducibility and accuracy were increased while costs were significantly reduced.
We offer assessing the feasibility of automating your methods with the accroma by testing your products on the system before purchase. We offer you a success guarantee meaning if the method transfer is not successful, you can give back the system and you will get the money back.
Find out today whether the accroma system can be applied for your samples and methods. Get valuable insights of how OOS events and hands on time can be significantly reduced. We will assist you in assessing accroma scientifically and economically. Contact us or book a demo without obligation.
Make sure you are the first person within your organization, which drives this initiative. Are you ready to improve accuracy while saving tremendous costs?

